Introducing a novel NaOH protective layer to improve sodium batteries

By introducing a NaOH protective layer, researchers have unveiled a novel approach to enhancing the stability and performance of sodium batteries, offering a scalable solution for the future of energy storage.
The quest for more efficient and durable energy storage solutions has led scientists to explore innovative methods to enhance sodium batteries. A recent study has introduced a NaOH protective layer as an easily scalable method to protect sodium without any additional chemicals or a special environment for this reaction. This achievement has the potential to significantly improve the stability and performance of sodium metal anodes.
Addressing sodium’s reactivity
Sodium, known for its high reactivity and tendency to form dendrites, poses significant challenges in battery applications. The formation of dendrites can lead to short circuits and reduced battery life. However, a team of researchers from the Leibniz Institute for Solid State and Materials Research (IFW) Dresden, in Germany, has discovered a simple yet effective solution: a NaOH protective layer. This layer, formed by exposing sodium to ambient conditions, mitigates these issues, ensuring a more stable and reliable performance.
Enhancing electrochemical stability
The study, published in the Journal of Energy Storage, highlights that the NaOH layer not only suppresses dendrite growth but also stabilizes the sodium metal anode against volume changes. This is crucial for maintaining the structural integrity of the anode during charge and discharge cycles. The protective layer acts as a mechanical barrier, preventing the sodium from reacting with the electrolyte solution and ensuring a consistent morphology.
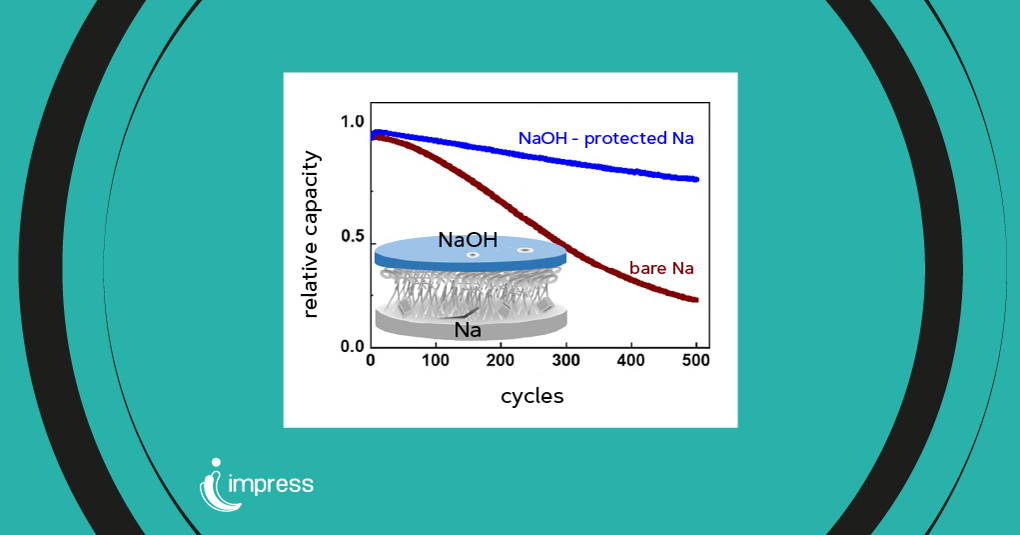
Prolonged battery life
In practical applications, the NaOH-protected sodium anode has demonstrated remarkable improvements in battery life. In symmetric Na-batteries, the protective layer increased the cell’s lifespan by eight times compared to non-protected sodium. Furthermore, in full-cell configurations with a layered sodium oxide cathode, the NaOH-protected anode retained 81% of its initial capacity after 500 cycles at a 1C current rate, a significant enhancement over the 20% capacity retention of non-protected anodes under the same conditions.
Scalability and practicality
One of the most compelling aspects of this innovation is its scalability. The method to form the NaOH protective layer is straightforward and does not require any additional chemicals or special environments. This makes it a practical solution for large-scale applications, potentially paving the way for more robust and reliable sodium batteries in the future.
The key benefits of the NaOH protective layer are:
- Suppresses dendrite growth on sodium metal anodes.
- Stabilizes the anode against volume changes during cycles.
- Increases the lifespan of sodium batteries significantly.
- Maintains high capacity retention in full-cell configurations.
- Scalable and practical for large-scale applications.
Toward advanced energy storage technologies
The introduction of the NaOH protective layer marks a significant milestone in the development of sodium batteries. By addressing critical issues such as dendrite formation and volume change, this innovation promises to enhance the stability and longevity of sodium metal anodes. As researchers continue to explore and refine this approach, the future of energy storage looks increasingly promising.
“The findings of our study fully exemplify the effectiveness of the interoperability approach which is at the core of the IMPRESS project. They demonstrate how innovative solutions like the NaOH protective layer can enhance the performance and stability of sodium batteries, paving the way for advanced energy storage technologies”, concludes Johannes Schultz, Research Scientist at Leibniz Institute for Solid State and Materials Research (IFW) Dresden and IMPRESS Project Partner.
Publication details
Journal of Energy Storage 77 (2024) 109900
NaOH protective layer for a stable sodium metal anode in liquid electrolytes
Alexander Thomas, Björn Pohle, Johannes Schultz, Martin Hantusch and Daria Mikhailova
doi: 10.1016/j.est.2023.109900